Purified Vero Cell Rabies Vaccine Validity, Https Academic Oup Com Cid Article Pdf 29 1 141 921370 29 1 141 Pdf
These are inactivated by ß-propriolactone and purified by ultracentrifugation Jaiiaroensup et al 1998. The next generation purified Vero cell rabies vaccine PVRV-NG is a highly purified vaccine.

Purified Vero Cell Rabies Vaccine Packaging Type Paper Box For Clinical Rs 290 Pack Id 14090049262
Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or.
Purified vero cell rabies vaccine validity. Purified Vero cell rabies vaccine was used to treat 19 patients who experienced. Purified Vero cell rabies vaccine PVRV contains inactivated and lyophilized Wistar strain of rabies virus grown on Vero cell cultures in fermenters allowing mass cultivation. Purified vero cell rabies vaccine validity.
2019-153 Please refer to Annex 2 for available photographs. Comparison of neutralizing antibody responses to post-exposure regimens - Volume 96 Issue 3. Postexposure therapy outside the United States may include materials not used in the US.
Two rabies vaccines are available in the United States. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The rabies vaccine should be administered in different injection site.
In this work we studied the purification of inactivated rabies virus produced in Vero cells grown in animal component free conditions using different methods. Product details are listed in Table 2 below and are also contained in the Philippines Food and Drug Administration Advisory No. Listing a study does not mean it.
31 If vaccine administered in less than 6 months of exposure cell culture rabies vaccine then one injection on day 0 is recommended. Comparison of Purified Vero Rabies Vaccine Serum Free With Human Diploid Cell Vaccine in Pre-exposure Use. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus and uses the Vero cell line as its host.
Listing a study does not mean it has been evaluated by the US. Among them Vero cell rabies vaccines which are used worldwide. Purified Vero cell rabies vaccine and human diploid cell strain vaccine.
CCEEVs use inactivated rabies virus grown from either embryonated eggs or in cell cultures and are safe for use in humans and animals. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose. Purified Vero cell rabies vaccine PVRV is a new effective but inexpensive tissue culture rabies vaccine for human use.
Verorab was licensed in 1985 for both pre- and post-exposure prophylaxis of rabies. Cell culture vaccines are recommended by World Health Organization WHO for pre and post exposure prophylaxis. A field trial of purified vero-cell tissue culture rabies vaccine PVRV was carried out and 566 children under 15 years of age were studied.
Rabies purified chick embryo cell vaccine is used to protect people who have been bitten by animals post-exposure or otherwise may be exposed to the rabies virus pre-exposure. PVRV Purified Vero cell Rabies Vaccine PVRV Pasteur Institute of India Coonoor Tamilnadu 4. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after.
CCEEVs can be used in both pre- and post-exposure vaccinations. PCECV vaccine RabAvert Novartis is produced in chick embryo cell culture. Purified vero cell rabies vaccine validity.
Rabies purified chick embryo cell vaccine is used to protect people who have been bitten by animals post-exposure or otherwise may be exposed to the rabies virus pre-exposure. HDCV vaccine Imovax Sanofi Pasteur is produced in human diploid cell culture. Rabipur Purified Chick Embryo Cell Vaccine PCECV Novartis Vaccines Mumbai 5 Rabivax Human Diploid Cell Culture Vaccine HDCV Liquid Serum Institute of India Pune 6 Vaxirab Purified Duck Embryo Vaccine PDEV Zydus Health Care ltd Ahmedabad.
The COVID-19 case fatality rate of 25 makes. Immunogenicity and safety of WHO-approved TRC-ID regimen with a chromatographically purified Vero cell rabies vaccine with or without rabies immunoglobulin in children. These are inactivated by ß-propriolactone and purified by ultracentrifugation Jaiiaroensup et al 1998.
We performed a phase II clinical study in adults in France to assess its immunological non-inferiority and clinical safety for pre-exposure prophylaxis. Study of Purified Vero Rabies Vaccine and Rabies Human Diploid Cell Vaccine in a Simulated Rabies Post-exposure Regimen The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Both types are considered equally safe and effective.
Such as purified vero cell rabies vaccine Verorab Imovax Rabies vero TRC Verorab purified duck embryo vaccine Lyssavac N or different formulations of PCEC Rabipur or. SPEEDA Purified Rabies Vaccines Vero Cell Falsified versions of 4 different combinations of batch numbers have so far been discovered. Epub 2014 Oct 15.
Epub 2018 Jan 10. Vaccination of subjects already fully immunized against rabies. 32 If vaccine administered in more than 6 months of exposure cell culture.
Both vaccines contain inactivated rabies virus. This vaccine works by exposing you to a small dose of the virus which causes the. Evolving rabies vaccination trends including shorter intradermal ID regimens with reduced volume along with WHO recommendation for ID administration has driven recent ID PVRV regimen assessments.
Safety and immunogenicity of chromatographically purified Vero cell rabies vaccine for intradermal pre- and post-exposure rabies prophylaxis. Read our disclaimer for details.
Https Www Mdpi Com 2414 6366 5 1 40 Pdf

Intradermal Post Exposure Rabies Vaccination With Purified Vero Cell Rabies Vaccine Comparison Of A One Week 4 Site Regimen Versus Updated Thai Red Cross Regimen In A Randomized Non Inferiority Trial In The Philippines Sciencedirect

Pdf Purified Vero Cell Rabies Vaccine And Human Diploid Cell Strain Vaccine Comparison Of Neutralizing Antibody Responses To Post Exposure Regimens
Https Www Who Int Vaccine Safety Initiative Tools Rabies Vaccine Rates Information Sheet Pdf
Speeda Full Prescribing Information Dosage Side Effects Mims Myanmar

Chromatographically Purified Vero Cell Rabies Vaccine Cprv Biovalys
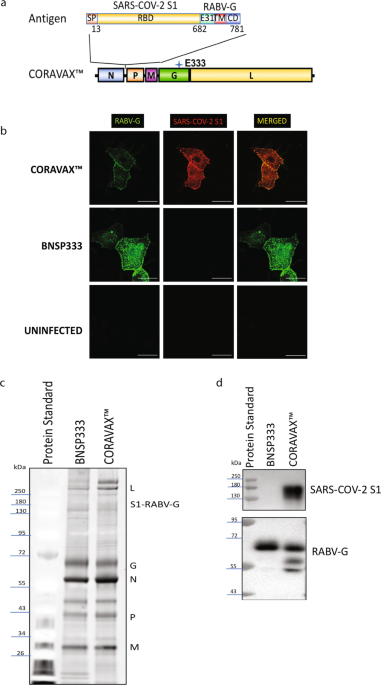
Rabies Virus Based Covid 19 Vaccine Coravax Induces High Levels Of Neutralizing Antibodies Against Sars Cov 2 Npj Vaccines
Abhayrab Rabies Vaccine United Pharmacies

Promising Rabies Vaccine For Postexposure Prophylaxis In Developing Countries A Purified Vero Cell Vaccine Produced In China Clinical And Vaccine Immunology
Https Www Tandfonline Com Doi Pdf 10 1080 14760584 2017 1294068

China Rabies Vaccine Vero Cell For Human Use Freeze Dried China Vaccine Human

Pdf Safety And Immunogenicity Of Purified Vero Cell Rabies Vaccine Versus Purified Chick Embryo Cell Rabies Vaccine Using Pre Exposure And Post Exposure Regimen Among Healthy Volunteers In San Lazaro Hospital
Https Www Mdpi Com 2414 6366 5 1 40 Pdf

Intradermal Post Exposure Rabies Vaccination With Purified Vero Cell Rabies Vaccine Comparison Of A One Week 4 Site Regimen Versus Updated Thai Red Cross Regimen In A Randomized Non Inferiority Trial In The Philippines Sciencedirect
Abhayrab Full Prescribing Information Dosage Side Effects Mims Philippines
Https Academic Oup Com Cid Article Pdf 29 1 141 921370 29 1 141 Pdf
China Products Of Rabies Vaccine Vero Cell For Human Use High Quality Products Of Rabies Vaccine Vero Cell For Human Use On Bossgoo Com

Medical Product Alert N 8 2019 Falsified Rabies Vaccines And Anti Rabies Serum

An Overview Of The Immunogenicity And Effectiveness Of Current Human Rabies Vaccines Administered By Intradermal Route Sciencedirect
