Sars Cov 2 Vaccine Vero Cell Inactivated Side Effects, Taiwan Puts All Eggs In One Basket By Refusing Mainland Vaccines Global Times
The primary outcome was efficacy against laboratory-confirmed symptomatic COVID-19 14 days following a second vaccine dose among participants who had no virologic evidence of SARS-CoV-2 infection at randomization. In conclusion we found that the inactivated SARS-CoV-2 vaccine BBIBP-CorV is tolerable and immunogenic in healthy people.
What Is Vero Cell Ema Launches Rolling Review Of Chinese Covid 19 Vaccine Euronews
2627 The ADE phenomenon has been reported in studies of Middle East respiratory syndromeCoV and SARS-CoV vaccines in animal challenge models.

Sars cov 2 vaccine vero cell inactivated side effects. From 14 days after the second dose to 14 days after the third dose the levels of neutralizing antibodies of these two groups continued to rise and this trend remained until 14 days after the fourth administration showing that the SARS-CoV-2 inactivated vaccine Vero cells could stimulate Sprague Dawley rats to produce a humoral immune response and the antibody is maintained at a high level for at least 2 weeks. The days 0 and 21 and days 0 and 28 two-immunisation schedules elicited significantly greater neutralising. Main outcomes and measures.
Here we report the pilot-scale production of an inactivated SARS-CoV-2 vaccine candidate BBIBP-CorV that induces high levels of neutralizing. Decision of the Secretary of Health. 151 In June 2021 a report revealed that the UB-612 vaccine developed by the US-based COVAXX was a venture initiated for profits by the Blackwater founder Erik Prince.
These might include swelling pain redness an itchy rash and other mild forms of. Vector virus vaccine based on influenza virus intranal spray in phase II trial in China since approx. Beijing Wantai Biological Pharmacy Xiamen University.
Inactivated SARS-CoV-2 produced with Vero cells since 2710. Rapid humoral responses against SARS-CoV-2 were noted from day 4 after the first inoculation and 100 seroconversion was found in all participants on day 42. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine.
Have a history of convulsion epilepsy encephalopathy or mental illness or family history. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. Named Carnivac-Cov it is an inactivated vaccine for carnivorous animals including pets aimed at preventing mutations that occur during the interspecies transmission of SARS-CoV-2.
West China Hospital Sichuan University. In January 2021 serum. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date.
Active Systemic Anaphylaxis No allergic reaction was observed after the guinea pigs was injected with high dosage vaccine intended for clinical use. Incidence of adverse events and reactions was collected among. The coronavirus disease 2019 COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 threatens global public health.
CoviVac KoviVac Chumakov Center Inactivated Unknown. Vaccine was administered alone to otherwise healthy control animals. However some of the side effects see section Side Effectsmay temporarily impact your ability to drive and use machines.
COVID-19 inactivated vaccine COVIran Barekat Shifa Pharmed Industrial Co Inactivated. SARS-CoV-2 Vaccine Vero Cell Inactivated CoronaVac for the prevention of COVID-19 July Updates Date of publication. Previous severe allergic reactions to vaccination such as acute allergic reactions urticaria dyspnea angioneurotic edema or abdominal pain or allergy to known ingredients of inactivated SARS CoV 2 vaccine have occurred.
Wait until these effects have worn off. SARS-CoV-2 Vaccine Vero Cells Minhai Biotechnology Co Inactivated Unknown. QazCovid-in QazVac Kazakhstan RIBSP Inactivated Unknown.
When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. Data from a phase 1 study suggested adequate safety and immunogenicity findings. CoronaVac Sinovac Inactivated 5038.
SARS-CoV-2 Vaccine Vero Cell Inactivated CoronavacOn 22 February 2021 the Food and Drug Administration FDA issued authorization granting IP BIOTECH INC. Conclusions and Relevance In this prespecified interim analysis of a randomized clinical trial treatment of adults with either of 2 inactivated SARS-CoV-2 vaccines significantly reduced the risk of symptomatic COVID-19 and serious adverse events were. The secondary outcome was efficacy against severe COVID-19.
The development of a vaccine is urgently needed for the prevention and control of COVID-19. 2627 However this was not observed in the preclinical study in an immunization-challenge model of rhesus macaques using the same vaccines. Pursuant to the role of the Health Technology Assessment Council HTAC to develop coverage recommendations particularly in the selection and financing of COVID-19 vaccines using the.
Bharat Biotech developed a Vero cell-based whole-virion inactivated SARS-CoV-2 vaccine BBV152 formulated with alum and a toll-like receptor 78 agonist producing a Th1-skewed response. CoronaVac has no known effect on the ability to drive and use machines. One concern about COVID-19 vaccines is the antibody-dependent enhancement ADE phenomenon that vaccine could make the subsequent SARS-CoV-2 infection more severe.
A person might also experience side effects around the injection site which is usually the upper arm. If you feel unwell do not drive or use machines. If later the vaccinated person comes into contact with SARS-CoV-2.
Inactivated Vero Cell Sinopharm Wuhan Inactivated 7251. In Phase II in China. The emergency use approval of SARS-CoV-2 Vaccine Vero Cell Inactivated CoronavacProduct InformationEnglishInformation on the safety of SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac is discussed in their comprehensive Risk Management PlanFor more information on reporting side.
Reproductive toxicity There was no effect on the fertility of parental female and male rats no toxicity and. BBV152 showed protection in non-human primate and hamster challenge models.
Chinese Citizens Are Already Receiving A Coronavirus Vaccine The New Yorker

Development Of An Inactivated Vaccine Candidate Bbibp Corv With Potent Protection Against Sars Cov 2 Sciencedirect
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Chinese Citizens Are Already Receiving A Coronavirus Vaccine The New Yorker
Covid Bolsonaro Hails Suspension Of Chinese Vaccine Trial Bbc News
Voa Twisted Words On Sinopharm Vaccine To Support Own Narrative To Discredit Chinese Products Expert Global Times

Full List Of Adverse Reactions From China S Sinopharm Vaccine Revealed Taiwan News 2021 01 11 09 17 00
China Injects Hundreds Of Thousands With Experimental Covid 19 Vaccines Wsj

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
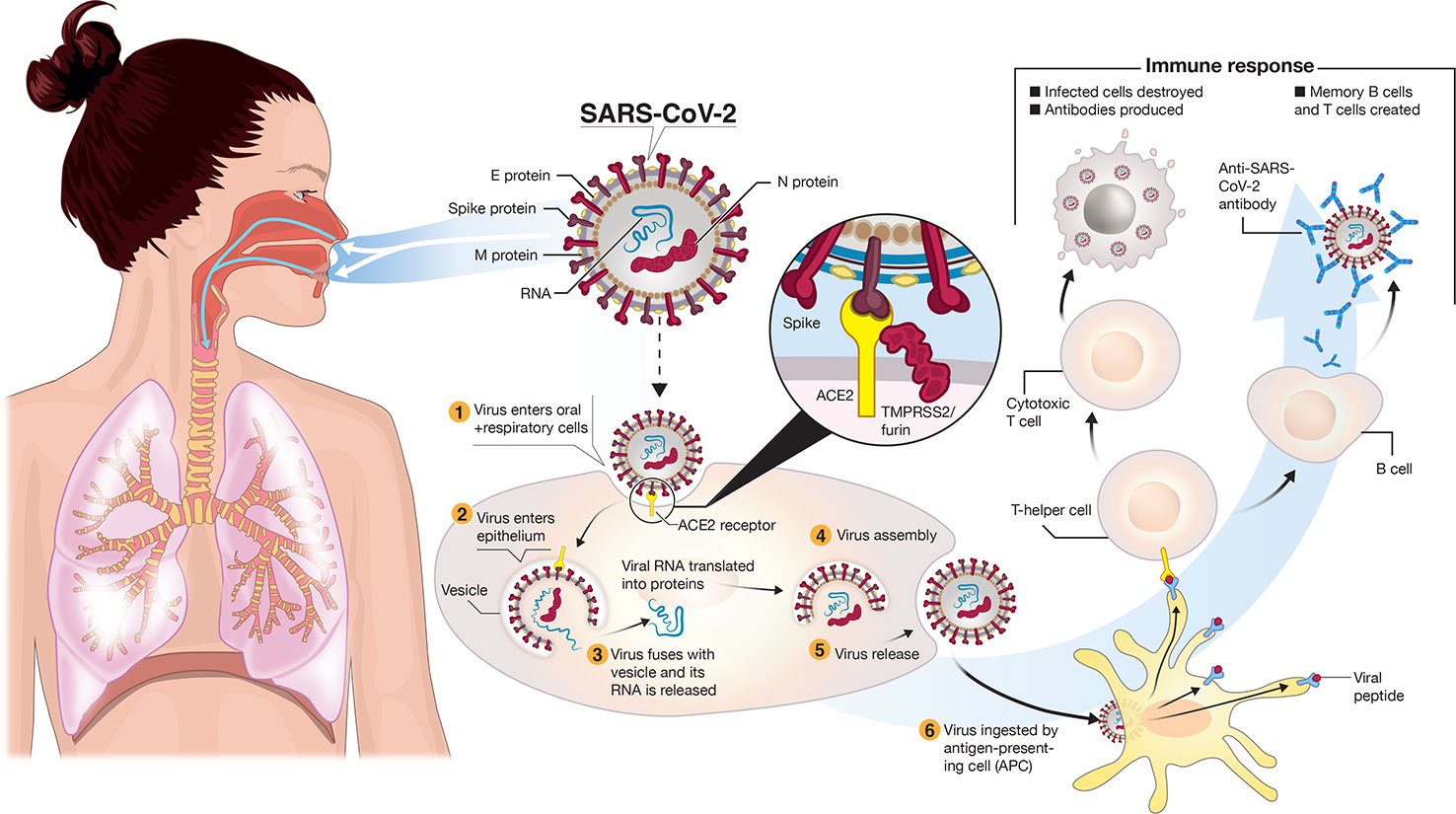
Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India
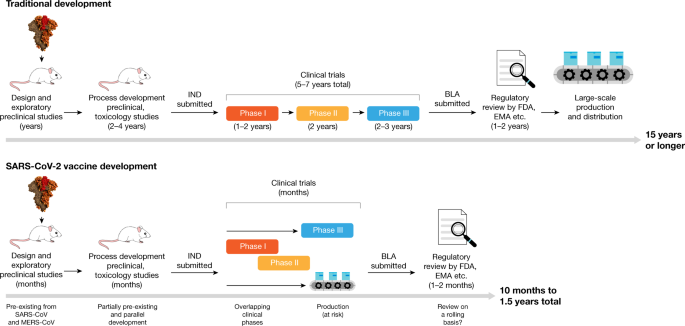
Sars Cov 2 Vaccines In Development Nature

Questions And Answers On Sinopharm Covid 19 Vaccine Bangkok Hospital

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases

Taiwan Puts All Eggs In One Basket By Refusing Mainland Vaccines Global Times

